304 stainless steel has good corrosion resistance and has been widely used. However, under the action of chloride ions, 304 stainless steel will undergo stress corrosion cracking. The #4 heater of Huadian Hongyanchi Power Generation Co., Ltd is a JR-2600-25-00 horizontal heater, which was officially put into operation in October 2018. In April 2019, abnormal water temperature fluctuations occurred in the heater, and it was found that the water hardness of the boiler system was seriously exceeding the standard. Immediately shut down the #4 heater in Station 1, and leaks occurred in 608 tube bundles. In order to further find out the cause of the leakage of the heat exchanger tube bundle and ensure the subsequent safe and stable operation of the heater, the leaking tube bundle was cut for test analysis.
1. Experiment
1.1 The macro situation of the cutting pipe
The sample tube is a cold-drawn seamless stainless steel tube made of 06Cr19Ni10 (S30408) with a size of φ19×1 mm. After appearance inspection and size measurement, no macroscopic visible cracks were found on the surface of the pipe, and no obvious thinning was found. There were only holes with uneven sizes and linear distribution. After the sample pipe is cut longitudinally, the inner wall is brown-red with a layer of sediment, and there are corrosion pits of uneven sizes and depth on one side accompanied by corrosion perforations. They are linearly distributed along the longitudinal direction of the pipe, which is consistent with the external macroscopic inspection results of the pipe. There is no uniform corrosion on the inner wall of the cleaned pipe section, but there are pitting corrosion pits and corrosion holes. The corrosion points expand from the inside to the outside as shown in Figure 1 (before cleaning) and Figure 2 (after cleaning). It can be seen from the figure that this pipe doesn't leak due to the accumulation of corrosion.

Figure 1 Before cleaning the inside of the cutting tube
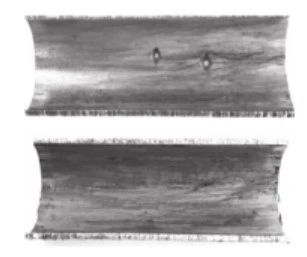
Figure 2 After cleaning the inside of the cutting tube
Table 1 Analysis results of main components of scale samples (%)
| Testing items | Fe2O3 | Cl- | NA2O | CaCO3 | Acid insoluble substance |
| Analysis results | 40.36 | 0.95 | 0.19 | 8.48 | 19.20 |
1.2 The mechanism of Cl-induced pitting corrosion of stainless steel
1.2.1 The theory of autocatalysis
The theory of autocatalysis is that the following processes exist on the surface of stainless steel:
Fe2++2H2O=Fe(OH)2+2H+, the presence of the acidic anion Cl- promotes the corrosion of stainless steel at the surface active parts. The pits formed by Fe+2H+=Fe2++H2 hinder the diffusion of H+ and help to maintain the acidic environment. The formation of new Fe2+ promotes the continuation of hydrolysis.
1.2.2 The theory of competitive adsorption
The competitive adsorption theory is that the adsorption and substitution of Cl- for oxygen leads to partial oxygen deficiency on the surface of the material, which hinders the oxidation of chromium on the surface of stainless steel. It is difficult to form a new passivation film, which leads to the destruction of the dynamic balance of the passive film of stainless steel. At the same time, Cl- Diffusion takes oxygen in the passivation film, which directly leads to the weakening of the stainless steel's passivation film. In conclusion, the presence of Cl- destroys the protective effect of the stainless steel film. The increase in temperature will lead to the acceleration of the autocatalytic effect and the enhanced destructiveness of the competitive adsorption of Cl-. The autocatalytic effect of the sudden temperature rises and the competitive adsorption and destruction of Cl- promote each other, which will make the stainless steel tube perforate rapidly.
1.3 The analysis of the scale
According to DL/T 1151-2012 "Analysis Methods of Scale and Corrosion Products in Thermal Power Plant" and DL/T 954-2005 "Water Vapor Test Method for Thermal Power Plant Trace Fluoride, Acetate, Formate, Chloride, Nitrous Acid, Radical Ion, Nitrate Ion and Phosphate Radical", analyze the composition of the scale sample on the inner wall of the sample tube; weigh 0.1g of the scale sample, and the test results are shown in Table 1.
There are 40.36% of Fe2O3 and 0.95% Cl- in the scale sample, and there are pits and perforations of different depths on the inner wall of the tube as shown in Figure 3 and Figure 4, indicating that there is an autocatalytic corrosion reaction on the inner wall surface of the heating tube; through observation and analysis, the microscopic morphology of the inner wall along the grain cracks in Figure 5 and Figure 6, and the intergranular cracks occur on the inner wall of the tube section in the circumferential direction, which conforms to the Cl-competitive adsorption theory, and destroys the stainless steel's protective film on the inner wall of the heating tube. Since the critical pitting temperature decreases significantly with the increase in Cl- concentration, whether from the autocatalysis theory or the competitive adsorption theory, the increase in ambient temperatures and Cl- concentration will lead to aggravated pitting corrosion of stainless steel. The direction of action of pitting corrosion is consistent.
Since there is a certain amount of Cl- in the circulating water inside the heater tube, if the ambient temperature does not change abruptly, the corrosion of the tube wall will proceed according to the regular corrosion law. The cause of this accident is that the wrong operation of the steam turbine on-duty staff provides an opportunity for corrosion of Cl-, and the amount of circulating water inside the tube bundle decreased, resulting in a relative increase in the Cl- concentration on the inner surface of the tube wall; the heating steam flow on the outer wall of the tube is not changed, resulting in a sudden rise in the temperature of the pipe wall, a rapid increase in the pitting rate of Cl- and perforation.
2. Conclusion
(1) The macroscopic appearance of the leaking pipe section is corrosion pits of different sizes and depths, accompanied by corrosion perforations. The inner diameter of the corrosion point is larger than the outer diameter, and it expands from the inside to the outside. The corrosion points are distributed in a line along the longitudinal direction of the pipe. Pitting features are obvious.
(2) The ability of 304 series stainless steel withstanding chloride ions is closely related to concentration and temperatures.
3. Recommendations
(1) The laboratory should regularly monitor the quality of circulating water and water plenishing in the heating pipes to ensure that the water quality meets the standard of circulating water in the heating pipe. Keep the inner surface of the heat exchange tube clean and free of deposits, and prevent pitting corrosion caused by chloride ions in the heat exchange tube.
(2) For materials of heat exchangers and the water quality of heating pipes, it is recommended to conduct an electrochemical test to study and determine the critical chloride ion concentration of pitting corrosion, so as to provide a basis for the control of the water quality of the heating pipe to ensure the safe operation of the heater.
(3) During the operation of the heating system, it is recommended to add high-temperature scale and corrosion inhibitor to the circulating water, which can not only prevent the scaling of the heat exchanger and inhibit the corrosion of the circulating water system, but also ensure the safe operation of the heating system.
(4) The choice of the maintenance of the heating system should be determined according to the actual situation. Dry protection measures should be adopted. After the water inside the heater tube bundle is completely drained, it should be rinsed with a certain pressure of demineralized water, and then dry one by one with compressed air. When wet maintenance is adopted, the changes in the pressure gauge should be checked regularly to prevent oxygen corrosion caused by air entering.